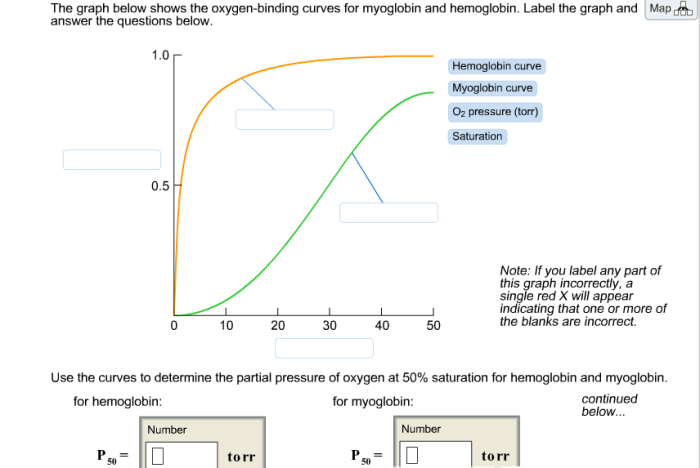
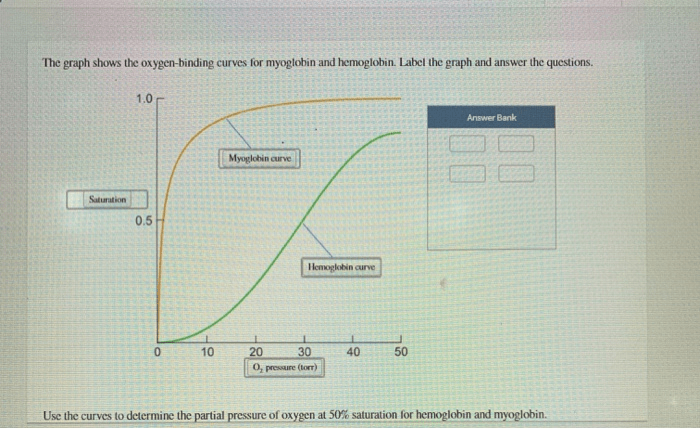
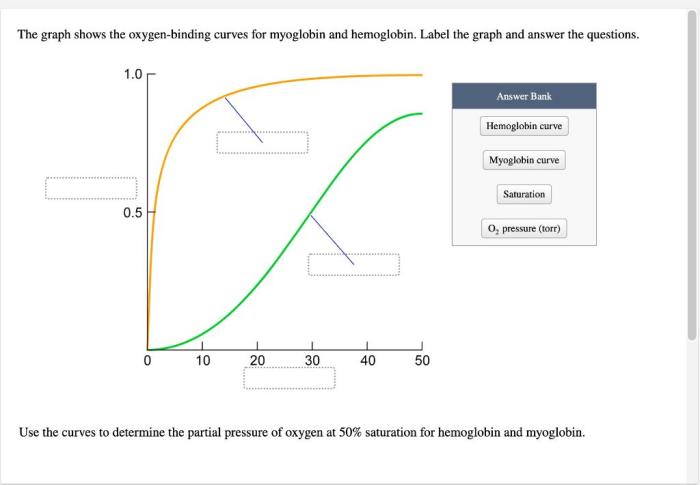
The graph shows the oxygen-binding curves for myoglobin and hemoglobin, two essential proteins involved in oxygen transport in biological systems. These curves provide insights into the molecular mechanisms underlying oxygen binding and the factors that modulate their affinities for oxygen.
This article delves into the structural and functional characteristics of hemoglobin and myoglobin, comparing their oxygen-binding properties and exploring their physiological implications.
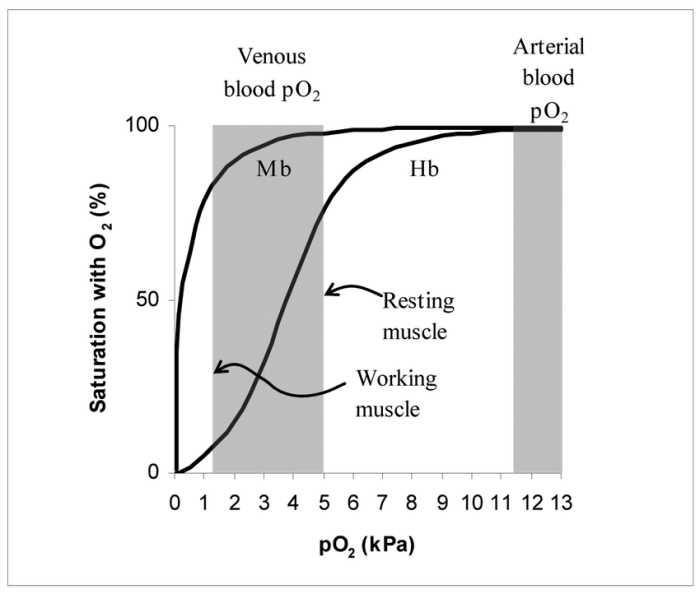
Hemoglobin, a complex protein found in red blood cells, exhibits cooperative oxygen binding, resulting in a sigmoidal oxygen-binding curve. Myoglobin, on the other hand, displays hyperbolic oxygen binding due to its lack of cooperativity. Understanding these differences is crucial for comprehending the distinct roles of these proteins in oxygen transport and utilization.
Oxygen-Binding Curves of Hemoglobin and Myoglobin: The Graph Shows The Oxygen-binding Curves For Myoglobin And Hemoglobin
Introduction
Oxygen-binding curves are essential for understanding the function of hemoglobin and myoglobin, two proteins responsible for oxygen transport in the body. These curves illustrate the relationship between the oxygen concentration and the percentage of oxygen-bound protein, providing insights into their oxygen-binding properties and physiological roles.
Hemoglobin
Hemoglobin is a complex protein found in red blood cells that transports oxygen from the lungs to the tissues. Its unique structure allows for cooperative binding of oxygen, resulting in a sigmoidal oxygen-binding curve.
Cooperative binding occurs when the binding of one oxygen molecule to hemoglobin increases the affinity of the remaining binding sites for oxygen. This results in a steeper slope of the oxygen-binding curve at higher oxygen concentrations, ensuring efficient oxygen delivery to tissues.
Allosteric effectors, such as hydrogen ions and carbon dioxide, can modulate hemoglobin’s oxygen affinity. Hydrogen ions decrease affinity, promoting oxygen release in tissues, while carbon dioxide increases affinity, facilitating oxygen uptake in the lungs.
Myoglobin
Myoglobin is a single-chain protein found in muscle cells that stores oxygen for use during muscle contraction. Unlike hemoglobin, myoglobin does not exhibit cooperative binding, resulting in a hyperbolic oxygen-binding curve.
The lack of cooperativity in myoglobin means that its oxygen affinity is constant, regardless of the oxygen concentration. This allows myoglobin to rapidly bind and release oxygen, matching the fluctuating oxygen demands of muscle cells during activity.
Comparison of Hemoglobin and Myoglobin, The graph shows the oxygen-binding curves for myoglobin and hemoglobin
| Feature | Hemoglobin | Myoglobin |
|---|---|---|
| Structure | Multi-subunit protein | Single-chain protein |
| Function | Oxygen transport | Oxygen storage |
| Oxygen-binding curve | Sigmoidal | Hyperbolic |
| Cooperativity | Present | Absent |
Hemoglobin’s cooperative binding and allosteric regulation make it well-suited for oxygen transport in the blood, where oxygen concentration varies significantly. Myoglobin’s constant oxygen affinity allows it to efficiently store and release oxygen in muscle cells.
Clinical Applications
Oxygen-binding curves are used in the diagnosis and monitoring of certain medical conditions. For example, anemia, a condition characterized by reduced hemoglobin levels, results in a decreased oxygen-binding capacity. Sickle cell disease, a genetic disorder, affects the shape of hemoglobin, altering its oxygen-binding properties.
Manipulating oxygen-binding curves has potential therapeutic applications. For instance, drugs that increase hemoglobin’s oxygen affinity could enhance oxygen delivery to tissues in conditions like heart failure or stroke.
FAQ Guide
What is the significance of oxygen-binding curves?
Oxygen-binding curves provide graphical representations of the relationship between oxygen partial pressure and the percentage of oxygen bound to a protein, such as hemoglobin or myoglobin. They help elucidate the molecular mechanisms of oxygen binding and the factors that influence protein affinity for oxygen.
How does cooperativity affect hemoglobin’s oxygen-binding curve?
Cooperativity in hemoglobin refers to the phenomenon where the binding of one oxygen molecule increases the affinity of neighboring subunits for oxygen. This results in a sigmoidal oxygen-binding curve, which is essential for efficient oxygen delivery to tissues.
What are the implications of myoglobin’s lack of cooperativity?
Myoglobin’s lack of cooperativity leads to a hyperbolic oxygen-binding curve. This means that myoglobin binds oxygen with a constant affinity, regardless of the oxygen concentration. This property enables myoglobin to store oxygen in muscles for use during periods of high energy demand.